Effik Polybactum® 9 eggs
- bvseo_sdk, p_sdk, 3.2.1
- CLOUD, getAggregateRating, 96.56ms
- REVIEWS, PRODUCT
- bvseo-msg: HTTP status code of 404 was returned;
Description
EFFIK
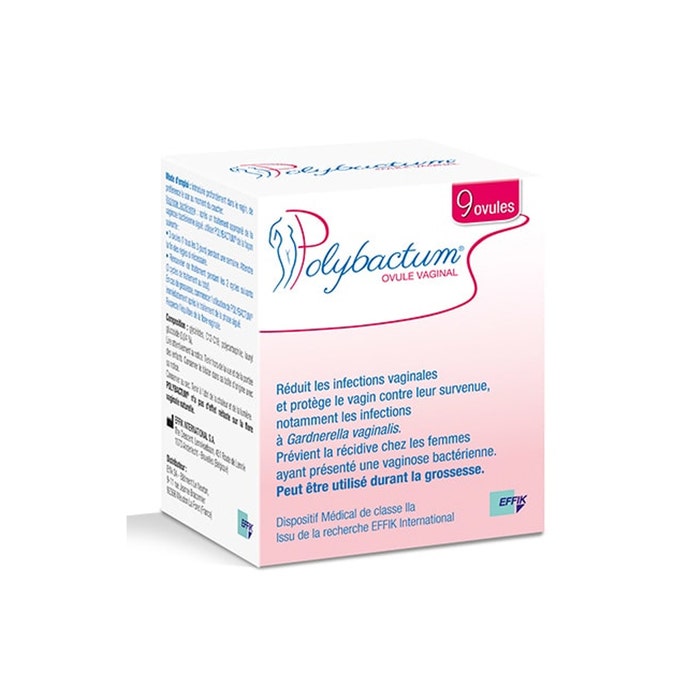
Polybactum®
Reduces vaginal infections and protects the vagina against their occurrence
In particular Gardnerella vaginalis infections.
Prevents recurrence in women who have had bacterial vaginosis
Class IIa medical device
9 ovules
Polybactum® is a product that adheres to the vaginal wall in such a way that it helps to reduce vaginal infections and protect the vagina against their occurrence (particularly those caused by Gardnerella Vaginalis) by creating a "barrier effect". It contains Polycarbophil (a mucoadhesive) and Lauryl Glucoside. Thanks to its mucoadhesive properties, Polybactum® inhibits the reconstitution of the biofilm produced by Gardnerella Vaginalis.
Polybactum® has a specific and selective bacteriostatic action, inhibiting the growth of Gardnerella Vaginalis while sparing the physiological flora. Polybactum® also has an acidifying effect on vaginal pH, encouraging the growth of lactobacillary flora.
In women with bacterial vaginosis, Polybactum® aims to prevent recurrence.
EFFIK
Meudon Business Park
Immeuble Le Newton
9-11 Rue Jeanne Braconnier
92 366 Meudon La Forêt Cedex
How to use it
Each ovum should be inserted deep into the vagina.
Bacterial vaginosis: after appropriate treatment of acute bacterial vaginosis, use Polybactum® as follows:
3 ova (1 every 3 days) for one week. Wait until the end of your period if necessary.
Repeat for the next 2 cycles (3 treatment cycles in total).
Compositions & ingredients
The composition may be subject to variations, so we advise you to always check the list on the purchased product.
Composition : Glycerides, C12-C18; Polycarbophil; Lauryl Glucoside.
Read the leaflet carefully. For Plus information, ask your doctor or pharmacist for advice.
This medical device is a healthcare product and, in accordance with these regulations, bears the CE mark.
Manufacturer: EFFIK International S.A.
Class IIa medical device. CE marking 0373.
Product for vaginal use only.
Do not use in the event of known hypersensitivity to one or more of the substances in the product's composition.
In the event of an unexpected reaction, discontinue use and consult a doctor.
Do not use concomitantly with other treatments administered vaginally.
Polybactum® may be used during pregnancy, but must be discontinued in the event of rupture of the membranes.
To avoid any interaction with other health products, you should always inform your doctor or pharmacist of any other treatment you are taking.
Condoms may be used during treatment.
The use of spermicidal substances and diaphragms is not recommended, as the risk of inactivating the contraceptive method cannot be excluded.
Keep out of sight and reach of children.
Do not use after the expiry date.
Keep the blister pack in its original box with the leaflet.
Keep dry. Keep away from heat and light.
Our selection
Reviews
- bvseo_sdk, p_sdk, 3.2.1
- CLOUD, getReviews, 8.27ms
- REVIEWS, PRODUCT
- bvseo-msg: HTTP status code of 404 was returned; HTTP status code of 404 was returned;
Questions
- bvseo_sdk, p_sdk, 3.2.1
- CLOUD, getContent, 95.33ms
- QUESTIONS, PRODUCT
- bvseo-msg: HTTP status code of 404 was returned;